发布时间:2021年01月29日 08:10:58 来源:振东健康网
全文翻译:菁菁
【摘要】前列腺原发癌肉瘤是一种罕见的侵袭性恶性肿瘤。我们报告了一名患者,他出现尿路阻塞症状,无放疗史,无既往腺癌史,且无雄激素剥夺治疗史。在尿道前列腺切除术后,依据组织病理学和免疫组化结果确诊为前列腺癌。本案例因其高度伪装性而被描述。
一、案例报告
一位有高血压和糖尿病病史的85岁男子,表现出提示尿路阻塞的体征和症状。临床检查以及实验室和放射学评估显示前列腺肥大,大小为28cm3,肾参数正常,血清前列腺特异性抗原(PSA; 3.1 ng/mL)正常。与麻醉师,内分泌科医生和心脏病专家协商后,计划进行尿道前列腺切除术(TURP)。在TURP期间,前列腺触感硬,并且切除过程与正常腺瘤切除不同。术后,病人症状减轻。
组织病理学结果显示,存在具有上皮和肉瘤成分的双相性肿瘤。上皮成分包括融合的、形态较差的腺体。腺体具有筛状特征且中央坏死,细胞显示出突出的核仁。肿瘤区域还具有梭形细胞和多形性细胞,其核细胞与癌性成分混合在一起(图1)。
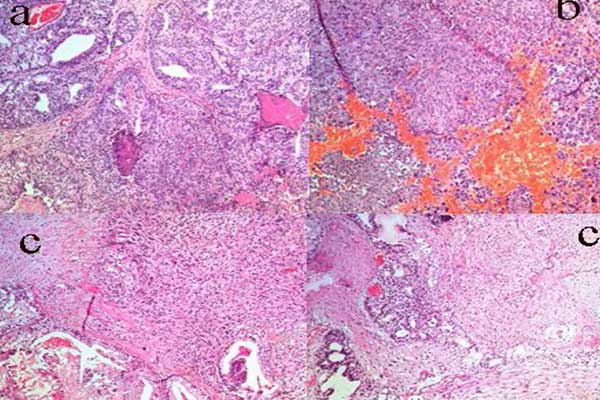
图1. 苏木精-伊红染色显示:(A)癌灶由融合的、具有筛状特征、中央坏死的不规则腺体组成;(B)梭形和多形性细胞的肉瘤区域和不典型的有丝分裂;(C)邻近肉瘤区域并有片状坏死的癌。
格里森分数为5+4=9,定为5级。免疫组化显示,癌灶中泛细胞角蛋白和波形蛋白呈阳性,癌变区域中泛细胞角蛋白、高分子量蛋白和NKX3.1呈阳性(图2,图3)。
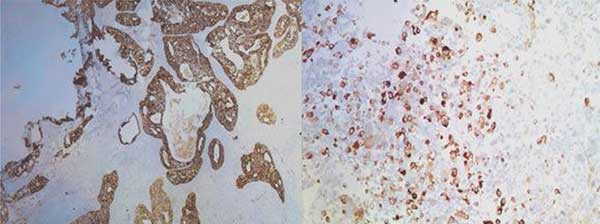
图2. 免疫组织化学染色显示癌细胞和肉瘤细胞中泛细胞角蛋白呈阳性。
临床医师诊断为前列腺癌(CSP)。组织学检查结果显示,患者应进行全身18F-氟脱氧葡萄糖(18F-FDG)正电子发射断层扫描(PET)。但是,由于COVID-19持续流行,该患者推迟了检查。在CSP诊断后将近6个月,患者进行了18F-FDG PET检查。
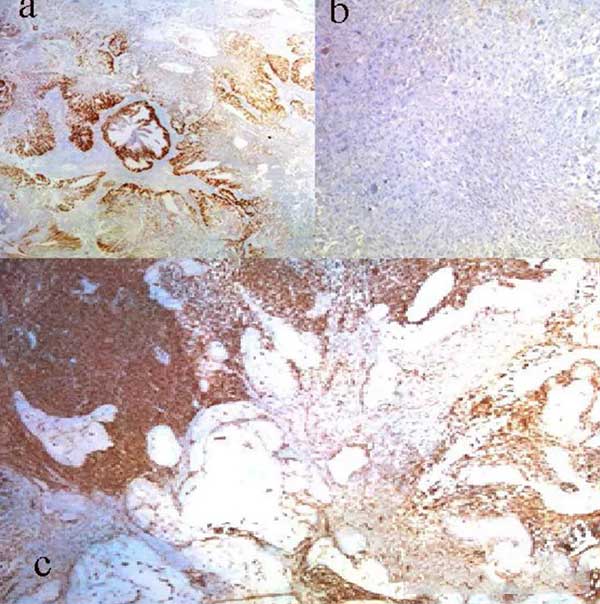
图3. 免疫组织化学染色显示(A)上皮细胞NKX3.1呈阳性;(B)在癌变区域中前列腺特异性抗原呈阴性;(C)肉瘤区波形蛋白呈阳性且腺成分呈阴性。
同时,由于激素疗法是唯一适用的治疗方案,患者开始接受激素治疗。在明确解释该疗法可能对他有效或无效(因为诊断是原发性CSP)后,治疗开始进行。多学科团队认为该患者应采用18F-FDG PET检查而不是磁共振成像(MRI)检查。
由于缺乏具体的经验,我们无法确定MRI在这种情况下是否有用。因为该患者的18F-FDG PET检查在进行CSP诊断后将近6个月才进行,很难确定手术前是否存在转移。但是,由于该患者的疾病状态本质上是转移性的,未对其进行局部放疗。考虑到患者的医疗状况,且辅助治疗的作用有限,患者在肿瘤委员会的共识下选择了定期随访。在提交此报告前,我们已经对患者进行了大约8个月的随访,他的身体状况较好。
二、讨论
原发性CSP在所有前列腺恶性肿瘤中占比少于0.1%[1]。它同时包含上皮和肉瘤样成分。肉瘤样成分可以是同源的或者异源的,包括骨肉瘤和软骨肉瘤等[2]。文献中记载的这一疾病的病例,大多数发生在生命的第七个或第八个十年。并且,多数患者有放疗史,或雄激素剥夺疗法史,或前列腺腺癌史。
CSP是一种罕见的恶性肿瘤,其特征是间充质和上皮成分混合。1967年,Hamlin和Lund[3]报告了首例CSP,该病的临床病程富有侵袭性,并且常与不良预后相关。
关于CSP的组织发生,有以下理论。主要包括偶然肉瘤和癌灶来自不同前列腺区域,未成熟干细胞的分化,腺癌向肉瘤形成的转化,激素和放射疗法后肿瘤的继发突变等[4]。根据文献,先前报告的病例通常与放射疗法或激素消融疗法史有关[5]。本次讨论的案例没有这样的治疗史。
CSP主要见于60岁以上的男性,最常见的症状是PSA正常的尿路阻塞。CSP常转移到肺部(43%),总生存率约为7个月。由于CSP是一种侵袭性疾病,因此强烈建议采用多种疗法。
Markowski等[6]对45例肉瘤样前列腺癌患者进行了10年期的回顾性生存分析。数据显示,局部疾病的生存结果优于全身疾病。然而,由于该疾病缺乏PSA表达,早期检测困难。因此,直肠指检在诊断这种罕见实体瘤中发挥重要作用。当前记录的疾病管理方案包括化学疗法,放射疗法,激素消融疗法,以及前列腺切除术[7,8]。然而,文献中未描述标准化的管理方案。
CSP是一种罕见的前列腺肿瘤,常伴有迅速恶化的临床病程。每个记录在案的病例都有助于研究病因、治疗选择和预后,并有助于预测影响总生存率的因素。由于血清PSA水平大多在正常范围内,直肠指检可能是早期发现的唯一方式。
【英文原文】
Carcinosarcoma of the Prostate Masquerading as Benign Prostatic Enlargement: Lesson Learned
【Abstract】Primary carcinosarcoma of the prostate is an extremely rare and aggressive malignancy. We report a patient who presented with obstructive symptoms and without a history of radiation, prior adenocarcinoma, or androgen deprivation therapy. Transurethral resection of the prostate was performed. Histopathology and immunohistochemistry revealed a confirmatory diagnosis of de novo carcinosarcoma of the prostate. The case is described for its rarity and masquerading nature.
1. Case Report
An 85-yr-old man with a history of hypertension and diabetes mellitus presented with signs and symptoms suggestive of urinary obstruction. Clinical examination and laboratory and radiological evaluations revealed firmfeeling prostatomegaly of 28 cm3 in size, normal renal parameters, and normal serum prostate-specific antigen (PSA; 3.1 ng/mL). After consulting with anesthetists, endocrinologists, and a cardiologist, transurethral resection of the prostate (TURP) was planned. During TURP, the prostate felt hard and the resection was different from a normal adenoma resection. The patient experienced relief of his symptoms. Histopathology revealed a biphasic tumor with epithelial and sarcomatous components. The epithelial component comprised fused, poorly formed glands with criominent nucleoli.
Areas of the tumor also had spindle and pleomorphic cells with enlarged hyperchromatic nuclei intermixed with the carcinomatous component (Fig. 1).

Fig. 1 Hematoxylin and eosin staining showing (A) carcinomatous focus composed of fused irregular glands with cribriform and central necrosis; (B)sarcomatous areas with spindle and pleomorphic cells and atypical mitosis; and (C) carcinoma with an adjacent sarcomatous area with patchy necrosis.
Grade group 5 with a Gleason score of 5 + 4 = 9 was assigned. Immunohistochemistry revealed positivity for pan-cytokeratin and vimentin in the sarcomatous foci, and positivity for pan-cytokeratin, high–molecular-weight keratin, and NKX3.1 in the carcinomatous areas (Figs. 2 and 3).

Fig. 2 – Immunohistochemical staining showing pan-cytokeratin positivity in carcinomatous and sarcomatous cells.
A diagnosis of carcinosarcoma of the prostate (CSP) was made. The histology findings prompted a recommendation for whole-body 18F-flurodeoxyglucose (18F-FDG) positron emission tomography (PET). However, because of the ongoing COVID-19 pandemic, the patient postponed the investigation and 18F-FDG PET was not performed until almost 6 mo after his CSP diagnosis.

Fig. 3 Immunohistochemical staining showing (A) NKX3.1 positivity in epithelial elements; (B) prostate-specific antigen negativity in carcinomatous areas; and (C) vimentin positivity in sarcomatous areas and negativity in the glandular component.
Meanwhile, hormone therapy was initiated because that was the only option available for the patient. This was commenced after a clear explanation that this therapy might or might not be effective for him as the diagnosis was primary CSP. The multidisciplinary team felt that it was appropriate to perform 18F-FDG PET rather than magnetic resonance imaging (MRI) for CSP.
We have no particular experience that allows us to comment on whether MRI would be useful in this setting. Since 18F-FDG PET was carried out almost 6 mo after CSP diagnosis and revealed metastatic disease, it was difficult to ascertain whether the metastasis was present before the surgery. However, as the disease state was metastatic in nature, local radiotherapy was not offered. Taking into account the patient’s medical profile and the limited role of adjuvant treatment, and with the consensus of the tumor board, the patient opted for regular follow-up. At the time of submission of this report, we have approximately 8 mo of follow-up for the patient and he is well and alive.
2. Discussion
Primary CSP accounts for <0.1% of all prostate malignancies [1]. It contains both epithelial and sarcomatoid components that can be homologous or heterologous, comprising osteosarcoma and chondrosarcoma, among others [2]. The majority of cases documented in the literature occurred in the seventh or eighth decade of life and patients had a history of radiotherapy, androgen deprivation therapy, or prostatic adenocarcinoma.
CSP is a rare malignant neoplasm that is characterized by an admixture of mesenchymal and epithelial components. In 1967, Hamlin and Lund [3] reported the first case of CSP, which has an aggressive clinical course and is associated with poor prognosis.
The following theories have been formulated regarding CSP histogenesis. These include the theory of incidental sarcomatous and carcinomatous foci arising from different prostatic areas, differentiation of immature stem cells,conversion to sarcoma formation from adenocarcinoma, and tumor differentiation secondary to hormonal and radiotherapy [4]. According to the literature, previously reported cases were commonly associated with a history of radiotherapy or hormonal ablation therapy [5]. The case discussed here had no such history.
CSP is predominantly seen in men aged >60 yr and the commonest symptom is urinary obstruction with normal PSA levels. CSP frequently metastasizes to the lungs (43%) and the overall survival rate is approximately 7 mo. Thus, as CSP is an aggressive disease, a multimodal approach is strongly recommended.
Markowski et al [6] carried out a retrospective survival analysis of 45 patients with sarcomatoid prostate carcinoma with 10-yr follow-up. The data revealed better survival outcomes for localized disease than for systemic disease. However, the lack of PSA expression makes early detection of the disease difficult. Thus, digital rectal examination plays a vital role in diagnosing this rare entity. The management options documented include chemotherapy, radiotherapy, hormone ablative therapy, and prostatectomy [7,8]. Nevertheless, no standardized management options have been described in the literature.
CSP is a rare prostatic neoplasm associated with a rapidly deteriorating clinical course. Each documented case helps in understanding the etiology, management options, and prognostic outcomes, and in predicting the factors influencing survival rates. Since serum PSA levels are mostly within the normal range, digital rectal examination may be the only option for early detection.
参考文献
[1]Aggarwal S, Mitra S, Dewan A, Durga G. Carcinosarcoma prostate with osteosarcomatous differentiation: a rare de novo presentation. BMJ Case Rep 2019;12:e230116.
[2]Somarelli JA, Boss MK, Epstein JI, Armstrong AJ, Garcia-Blanco MA.Carcinosarcomas: tumors in transition? Histol Histopathol 2015;30:673–87.
[3]Hamlin WB, Lund PK. Carcinosarcoma of the prostate: a case report. J Urol 1967;97:518–22.
[4]Lauwers GY, Schevchuk M, Armenakas N, Reuter VE. Carcinosarcoma of the prostate. Am J Surg Pathol 1993;17:342–9.
[5]Dundore PA, Cheville JC, Nascimento AG, et al. Carcinosarcoma of the prostate. Report of 21 cases. Cancer 1995;76:1035–42.
[6]Markowski MC, Eisenberger MA, Zahurak M, et al. Sarcomatoid carcinoma of the prostate: retrospective review of a case series from the Johns Hopkins Hospital. Urology 2015;86:539–43.
[7]Fukawa T, Numata K, Yamanaka M, et al. Prostatic carcinosarcoma: a case report and review of the literature. Int J Urol 2003;10:108–13.
[8]Subramanian VS, Coburn M, Miles BJ. Carcinosarcoma of the prostate with multiple metastases: case report and review of the literature. Urol Oncol 2005;23:181–3.
