发布时间:2021年02月21日 09:02:18 来源:振东健康网
节选翻译:振东文宣编辑部/菁菁
本文初次发表在2020年医学学术期刊《自然》,文献表明脂质滴参与了一线细胞内防御。它们充当的角色是先天免疫分子的开关。它不仅可以吸引病原体,还可以协调针对不同种类的病原体同时运行的不同免疫系统。
本文作者:Marta Bosch, Miguel Sánchez-Álvarez, Alba Fajardo, Ronan Kapetanovic, Bernhard Steiner,Filipe Dutra, Luciana Moreira, Juan Antonio López, Rocío Campo, Montserrat Marí,Frederic Morales-Paytuví, Olivia Tort, Albert Gubern, Rachel M. Templin, James E. B. Curson,Nick Martel, Cristina Català, Francisco Lozano, Francesc Tebar, Carlos Enrich,Jesús Vázquez, Miguel A. Del Pozo, Matthew J. Sweet, Patricia T. Bozza,Steven P. Gross, Robert G. Parton, Albert Pol
哺乳动物的脂质滴是先天免疫枢纽,可以整合细胞代谢和宿主防御
【摘要】引言:脂质滴(LD)存在于所有的真核细胞中。脂质滴可以存储和提供脂质,脂质则支持信号分子的产生,膜结构的构成,以及代谢能量的生成。脂质滴单层还能容纳和脂质没有明显关系的蛋白质,例如转录因子、染色质组分、有毒蛋白质。常见的寄生虫(如:锥虫、恶性疟原虫)、细菌(如:分歧杆菌、衣原体)和病毒(如:丙型肝炎、登革热)会在生命周期中诱导靶向脂质滴。此前的观点是,脂质滴为微生物提供生长基质,从而协助感染。
原因:成功的先天防御对于生存至关重要。随着病原体的进化,宿主物种也不断进化,产生了大量的免疫机制。许多线索表明,包含细胞应激、与危险模式相关的分子模式,如脂多糖,可诱导脂质滴形成。因此,脂质滴的位置和动态,可能在组织细胞内宿主防御中发挥积极作用。我们的研究表明哺乳动物脂质滴在先天免疫中具有直接作用和协调作用。
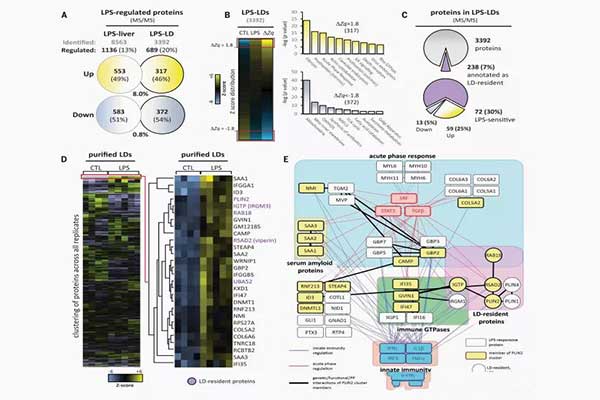
结果:研究表明,哺乳动物的脂质滴具有蛋白质介导的抗菌功能。在微生物败血症过程中,脂多糖促使脂质滴上调。光镜和电子显微镜显示,人巨噬细胞中脂质滴与细菌特异性结合,表明存在促进抗菌脂质滴蛋白与细菌结合的对接机制。一项质谱分析表明,因脂多糖-脂质滴(LPS-LDs)反应而引起的蛋白质差异,揭示了细胞器蛋白质组的深度重塑。经过严格评估,研究确定689种蛋白质受到LPS-LDs的调节,并发生变化(317个增强,372个减少)。创新途径分析显示,先天免疫系统相关成分更加丰富,而新陈代谢相关的脂质滴驻留蛋白减少。进一步分析表明,脂质滴是先天免疫中枢,整合了主要的细胞内和细胞外免疫反应。在五种脂质滴表面蛋白中,PLIN5是唯一一个因LPS-LDs作用而下调的。PLIN5的下调促进了LPS-LDs与线粒体在物质上和功能上的分离。同时,这一过程伴有氧化代谢和生酮作用的降低。迫使PLIN5重新表达,则导致了脂质滴-线粒体接触增加,脂质滴-细菌的相互作用减少,并损害了细胞的抗菌能力。
相比之下,PLIN2是LPS-LDs上上调最多的PLIN。基因相互作用分析表明,多种免疫蛋白对脂多糖作出反应,从而在PLIN2周围成核。LPS-LDs对几种干扰素诱导的蛋白产生刺激,如viperin, IGTP, IIGP1, TGTP1, 和 IFI47。进一步来说,LPS-LDs也导致了金环蛇抗菌肽(CAMP)的积聚。这是一种具有趋化特性的抗菌肽。如果细胞过量表达了与脂质滴相关的CAMP,则对不同细菌具有更高的抵抗力。这些细菌包括大肠杆菌,耐甲氧西林的金黄色葡萄球菌和单核细胞增生李斯特菌。
这些结果表明,脂质滴参与了一线细胞内防御。它们充当的角色是先天免疫分子的开关。通过重塑细胞代谢和引发蛋白质介导的抗菌机制,来对危险信号作出反应。本研究观察并描述了针对几种病原体的脂质滴转运,与吞噬细胞和寄生虫膜对接的机制,有可能有助于传递位于脂质滴表面的免疫蛋白。细胞内脂质滴可以为受到感染的细胞带来许多好处。它不仅可以吸引病原体,还可以协调针对不同种类的病原体同时运行的不同免疫系统。脂质滴还可以隔离细胞毒性化合物(例如,抗菌肽),从而减少其对其他细胞器的损害。鉴于目前抗生素面临广泛耐药性,本研究有助于破译参与抗菌防御的分子机制,为开发新型抗感染药提供参考。
1、引言
脂质滴是真核细胞脂质的主要存储细胞器。常见的寄生虫(例如锥虫和恶性疟原虫),细菌(例如分歧杆菌和衣原体)和病毒(例如丙型肝炎病毒和登革热)会在生命周期中被诱导并靶向脂质滴。当前的观点是,脂质滴能够支持感染,为入侵者提供生存和/或生长的基质。
然而,成功的先天防御对于宿主生存至关重要。宿主的免疫反应已经与病原体共同进化,并形成了多种防御机制。初步证据表明,脂质滴在先天防御过程中发挥了积极作用。例如,三种与免疫系统相关的先天性免疫蛋白存在于被感染细胞的脂质滴。①毒蛇蛇毒。它可以对抗在脂质滴上组装的两种病毒,HCV和DENV.②干扰素-g(IFN-g)诱导的鸟苷三磷酸酶(GTPase)(IGTP)。它是宿主抵抗弓形虫所必需的。③脂质滴上的组蛋白。在果蝇胚胎受到细菌攻击的情况下,它能够增加果蝇胚胎的存活率。本研究分析了哺乳动物的脂质滴在免疫防御中是否具有直接作用或调节作用。
由于所有的真核细胞都积累了脂质滴,所以这种先天的防御机制可能无处不在。因此,它可以作为治疗性干预的合适靶点。
2、结果
哺乳动物脂质滴显示出抗菌活性,这种活性由受到调节的蛋白介导。我们选择肝脏脂质滴来证明哺乳动物脂质滴参与先天免疫。肝脏调节全身的免疫反应,因而脂质滴相关病原体靶向肝脏脂质滴。我们在大肠杆菌的细菌杀灭试验中检测了肝脏脂质滴蛋白的抗菌能力。该细菌是肠道微生物群的重要组成部分,是引起严重临床感染的原因。
首先,我们给小鼠注射脂多糖。脂多糖是先天免疫的激活剂。由于经脂多糖处理的动物(LPS-小鼠)减少了食物摄入,因此对脂多糖-小鼠进行了额外禁食,并与同样禁食、注射盐水缓冲液的小鼠(CTL-小鼠)进行了比较。尽管透射电镜观察到两种治疗方法下,脂质滴形态差异明显,但两种处理方式均导致了相似的肝甘油三酸脂水平(图1,A,B和C)。尽管LPS-LDs小鼠较小,经脂多糖处理肝脏的LPS-LDs小鼠中,脂质滴数量高于CTL-脂质滴小鼠。(图1,D和E)。CTL-LD和LPS-LDs均被纯化(图1F),脂质滴蛋白和大肠杆菌一起培育。依据菌落的单位(CFUs),本研究评估了细菌生长情况。脂质滴蛋白减少了细菌生长,且LPS-LDs蛋白具有更高的抗菌能力(图1G)。
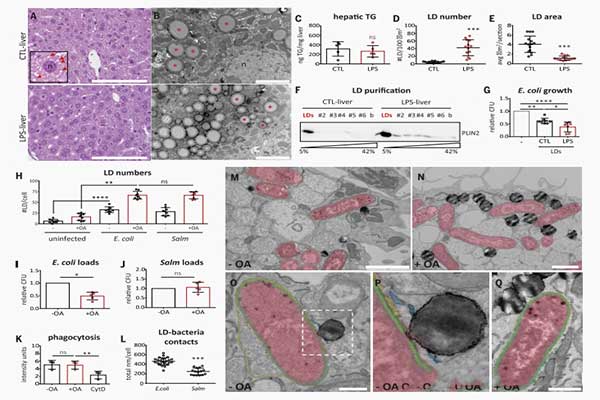
使用饲喂小鼠的脂质滴蛋白,通过悬浊液培养,研究证实了LPS-LDs蛋白的较强抗菌能力。为了确定脂质滴的抗菌活性,研究使用盲肠结扎穿孔术(CLP)获取小鼠肝脏脂质滴,并据此建立了多菌败血症模型。与CTL-LDs相比,CLP-LDs蛋白具有更强的抗菌能力。因此,研究表明哺乳动物Lan LDs具有蛋白质介导的抗菌能力,且该能力与感染相关。接下来,我们分析了脂质滴是否能减少人单核细胞衍生巨噬细胞(HMDM)中细菌的生长,HMDM来自健康捐献者。油酸是一种能够有效酯化为脂质滴的脂肪酸。在HMDM中,使用油酸孵育加快了脂质滴的积累。未经处理的、载有脂质滴的HMDM被非致病性大肠杆菌感染,或被专门的巨噬细胞内病原体,鼠伤寒沙门菌感染。HMDM通过增加脂质滴数量来应对感染(图1H)。在载有脂质滴的HMDM中,大肠杆菌的存活率(图1I)降低,但吞噬能力未降低。
与之相反的是,脂质滴不会降低鼠伤寒沙门菌的存活率(图1J),这与该病原体逃避抗菌免疫的能力保持一致。在受大肠杆菌感染的巨噬细胞中,脂质滴通常位于细菌附近(图1,M至Q)。比较分析表明,与鼠伤寒沙门菌相比,脂质滴与大肠杆菌之间作用更加频繁、作用时间更长(图1L)。在HMDM中,脂质滴与大肠杆菌的接触部分增加。
TEM分析显示,在脂质滴与大肠杆菌接触的部位,脂质滴单层(含有脂质滴蛋白)在细菌液泡膜中产生明显的间断,并且可能与细菌周质相互作用(图1,O至Q)。因此,位于脂质滴上的巨噬细胞显示出更强的抗菌能力。这表明存在一种对接机制,该机制能够促进抗菌脂质滴蛋白与细菌的结合。
3、讨论
病原体需要宿主衍生的脂质来维持其生命周期,而脂质滴提供了这些脂质。同时,脂质滴的潜在能力是参与针对细胞内病原体的有效免疫。结果显示,至少30%的脂质滴蛋白对脂多糖敏感。这表明,先天免疫系统具备包括了大量由脂质滴重塑的免疫机制。研究分析表明,免疫相关蛋白的复杂基因簇在受感染细胞的脂质滴上进行组织。除了先前描述的脂质滴常驻免疫蛋白(例如毒蛇毒素和IGTP),我们还鉴定了IIGP1,TGTP1和IFI47。我们的分析还确定了CAMP是在脂质滴上发挥作用的有效抗菌蛋白。这些蛋白质可以以协同方式单独发挥作用,和/或协同杀死病原体。
试验观察并描述了几种病原体的脂质滴如何运输、吞噬,及如何与寄生虫细胞膜对接。着可能有助于脂质滴表面上免疫蛋白的传递。蛋白质在脂质滴上积累,从而提升了稳定性。并且,脂质滴限制了潜在的细胞毒性肽,防止损伤其它细胞。研究表明,脂多糖触发了脂质滴和线粒体的物理分离,这一过程的原因或部分原因,是LPS-LD上PLIN5水平降低。解偶联可能既反映了自我保护程序(避免了线粒体损害),也反映了如何将可与细菌相互作用的脂质滴数量最大化。同时,减少的脂质滴-线粒体相互作用可能导致独特的免疫代谢特征:(i)线粒体介导的脂质滴消耗减少导致宿主脂质滴的积累;(ii)由于脂肪酸氧化减少,被感染细胞显示的OXPHOS减少;(iii)受感染的动物显示出较低的生酮率。
这些研究强调,哺乳动物脂质滴构成细胞内第一道防线。脂质滴积极参与至少两个水平的先天免疫反应,它促进抗菌蛋白积累、发挥作用,并且调节免疫细胞的代谢。由于在病原体中普遍存在对当前抗生素的广泛耐药性,因此,在目前看来,引起脂质滴介导的细胞防御机制可能会为新的抗感染疗法、策略提供依据。
英文节选:
Mammalian lipid droplets are innate immune hubs integrating cell metabolism and host defense
【摘要】Introduction: In all eukaryotic cells, lipid droplets (LDs) store and supply essential lipids to produce signaling molecules, membrane building blocks, and metabolic energy. The LD monolayer also accommodates proteins not obviously related to lipids, such as transcription factors, chromatin components, and toxic proteins.Common parasites (such as trypanosomes and Plasmodium falciparum), bacteria (such as mycobacteria and Chlamydia), and viruses (such as hepatitis C and dengue) induce and target LDs during their life cycles. The current view is that LDs support infection, providing microorganisms with substrates for effective growth.
Rationle: Successful innate defense is critical for survival, and host species have efficiently coevolved with pathogens to develop a plethora of immune responses. Multiple cues, including cellular stress and danger-associated molecular patterns such as lipopolysaccharide (LPS), induce LD formation. Thus, LD localization and dynamics may potentially be advantageous for organizing an intracellular host defense. We have investigated the possibility that mammalian LDs have a direct and regulated role in innate immunity.
Results: We show that mammalian LDs are endowed with a protein-mediated antimicrobial capacity, which is up-regulated during polymicrobial sepsis and by LPS. Light and electron microscopy demonstrated specific association of LDs and bacteria in human macrophages, suggesting the existence of docking mechanisms that facilitate the engagement of antibacterial LD proteins with bacteria. A comparative mass spectrometry profiling of proteins differentially associated with LDs in response to LPS (LPS-LDs) revealed the profound remodeling of the organelle proteome.
A stringent evaluation identified 689 proteins differentially regulated on LPS-LDs (317 enriched and 372 reduced). Ingenuity Pathway Analysis revealed an enrichment of innate immune system–related components and reduction of metabolism-related LD-resident proteins. Additional analyses suggested that LDs serve as innate immune hubs, integrating major intra- and extracellular immune responses.
Among the five members of the perilipin family of LD surface proteins (PLINs), PLIN5 was the only one down-regulated on LPS-LDs. PLIN5 reduction promoted physical and functional disconnection of LPS-LDs and mitochondria, with a concomitant reduction of oxidative metabolism and ketogenesis. Forced PLIN5 reexpression increased the number of LD-mitochondria contacts, reducing LD-bacteria interactions and compromising the antimicrobial capacity of cells.
By contrast, PLIN2 was the most up-regulated PLIN on LPS-LDs. Gene interaction analysis revealed that multiple immune proteins nucleated around PLIN2 in response to LPS. LPS-LDs accrued several interferon-inducible proteins such as viperin, IGTP, IIGP1, TGTP1, and IFI47. Furthermore, LPS-LDs also accumulated cathelicidin (CAMP), a broad-spectrum antimicrobial peptide with chemotactic properties. Cells overexpressing a LD-associated CAMP were more resistant to different bacterial species, including Escherichia coli, methicillin-resistant Staphylococcus aureus, and Listeria monocytogenes.
Conclusion: These results demonstrate that LDs form a first-line intracellular defense. They act as a molecular switch in innate immunity, responding to danger signals by both reprogramming cell metabolism and eliciting protein-mediated antimicrobial mechanisms. Mechanisms of LD trafficking and docking with phagocytic and parasitophorous membranes, observed here and described for several pathogens, may facilitate the delivery of immune proteins located on the LD surface. Intracellular LDs can provide infected cells with several biological benefits, serving as a location to attract pathogens as well as coordinating different immune systems that operate simultaneously against different classes of pathogens. LDs may also sequester cytotoxic compounds (such as antimicrobial peptides), reducing damage to other cellular organelles. In view of the widespread resistance to current antibiotics, this study helps decipher molecular mechanisms involved in antimicrobial defense that could be exploited for development of new anti-infective agents.
Introduction
Lipid droplets (LDs) are the major lipid storage organelles of eukaryotic cells. Common parasites (such as trypanosomes and Plasmodium falciparum), bacteria (such as mycobacteria and Chlamydia), and viruses [such as hepatitis C (HCV) and dengue (DENV)] induce and target LDs during their life cycles. The current view is that LDs support infection, providing invaders with substrates for survival and/or growth.
However, successful innate defense is critical for survival, and host immune responses have coevolved with pathogens, developing a plethora of defense mechanisms. There is some limited evidence that LDs actively participate in innate defense. For example, three innate immune system–related proteins localize to the LDs of infected cells: (i) viperin, which is active against two viruses assembled on LDs (HCV and DENV); (ii) interferon-g (IFN-g)–inducible guanosine triphosphatase (GTPase)(IGTP), which is required for resistance to Toxoplasma gond(ii); and (iii) histones on LDs, which increase the survival of bacterially challenged Drosophila embryos. We analyzed whether mammalian LDs have a direct or regulated role in immune defense.
Because all eukaryotic cells accumulate LDs, this innate defense mechanism may be ubiquitous and therefore serve as a suitable target for therapeutic intervention.
Results
Mammalian LDs display regulated protein-mediated antibacterial activity. We selected hepatic LDs as a proof of concept that mammalian LDs participate in innate immunity. The liver modulates the systemic immune response, and hepatic LDs are targeted by LD-related pathogens. We tested the antibacterial capacity of hepatic LD proteins in a bacterial killing assay of Escherichia coli, an abundant component of the intestinal microbiota and cause of serious clinical infections.
First, we injected mice with lipopolysaccharide (LPS), an activator of innate immunity. Because LPS-treated animals (LPS-mice) reduce food intake, LPS-mice were additionally fasted and compared with mice injected with saline buffer and identically fasted (CTL-mice).
Both treatments promoted similar hepatic triglyceride levels (Fig. 1, A, B, and C), although morphological differences between LDs were evident from transmission electron microscopy (TEM). The number of LDs in LPS-treated livers (LPS-LDs) was higher than in those of fasted animals (CTL-LDs), although LPS-LDs were smaller (Fig. 1, D and E). CTL- and LPSLDs were purified (Fig. 1F), and LD proteins were incubated with E. coli. Bacterial viability was estimated from the resulting colony-forming units (CFUs). LD proteins reduced bacterial growth, and LPS-LD proteins demonstrated enhanced antibacterial capacity (Fig. 1G). This enhancement was confirmed in suspension cultures and by use of LD proteins from fed mice.
To determine LD antibacterial activity during an actual infection, mouse liver LDs were obtained after cecal ligation and puncture (CLP), a model of polymicrobial sepsis. CLP-LD proteins exhibited enhanced antibacterial capacity when compared with CTL-LDs. LPS and CLP-LD proteins reduced bacterial growth even after a shorter incubation time. Bacterial growth was unaffected by oleic acid (OA), the major fatty acid component of hepatic LDs, or by cytosolic proteins from CTLand LPS-livers. Thus, mammalian LDs have a protein-mediated antibacterial capacity, which is regulated by infection.
Next, we analyzed whether LDs reduce bacterial growth in human monocyte-derived macrophages (HMDMs) from healthy donors. In HMDMs, LD accumulation was promoted by incubation with OA, a fatty acid efficiently esterified into LDs. Untreated and LD-loaded HMDMs were infected with either nonpathogenic E. coli or the professional intramacrophage pathogen Salmonella enterica serovar Typhimurium (Salm). HMDMs responded to infection by increasing LD numbers (Fig. 1H). E. coli survival (Fig. 1I), but not phagocytic capacity (Fig. 1K), was reduced in LD-loaded HMDMs.
By contrast, LDs did not reduce Salm survival (Fig. 1J), which is in keeping with this pathogen’s ability to avoid antimicrobial responses. In E. coli–infected macrophages, LDs were often in the proximity of bacteria (Fig. 1, M to Q). Comparative analyses demonstrated that LDs were closer to and more frequently established longer contacts with E. coli than with Salm (Fig. 1L). These LD–E. coli contact sites increased in loaded HMDMs.
TEM analysis revealed that in LD–E. coli contact sites, the LD monolayer (containing LD proteins) produced an apparent discontinuity in the bacterial vacuolar membrane and probably interacted with the bacterial periplasm (Fig. 1, O to Q). Thus, LD-loaded macrophages display enhanced antibacterial capacity, which suggests the existence of docking mechanisms that enable or facilitate the engagement of antibacterial LD proteins with bacteria.
Discussion
Pathogens require host-derived lipids to support their life cycles, with LDs providing a source of these lipids. As a result, LDs also have the potential to deliver effective host defenses against intracellular pathogens. We show that at least 30% of the LD proteome is LPSsensitive, suggesting that innate immunity has developed a host defense program that includes extensive LD remodeling. Our analyses demonstrate that complex clusters of immunityrelated proteins organize on LDs of infected cells. In addition to previously described LDresident immune proteins, such as viperin and IGTP, we have identified IIGP1, TGTP1, and IFI47. Our analysis also identified CAMP as a professional antibacterial protein efficiently functioning on LDs. These proteins may act individually, in a coordinated manner, and/or synergistically to kill pathogens.
Mechanisms of LD trafficking and docking with phagocytic and parasitophorous membranes, observed here and described for several pathogens, may facilitate the delivery of immune proteins located on the LD surface. Accumulation on LDs may provide stability to these proteins and may restrict these potentially cytotoxic peptides to LDs, preventing indiscriminate cellular damage. In this respect, we have shown that LPS triggers physical separation of LDs and mitochondria, at least partly because of reduced PLIN5 levels on LPSLDs. Uncoupling likely reflects both a selfprotection program (to avoid mitochondrial damage, in view of their prokaryotic evolutionary origin) and a means to maximize or increase the number of LDs available to interact with bacteria. Simultaneously, the reduced LD-mitochondria interaction may lead to distinctive immunometabolic features: (i) the accumulation of host LDs, resulting from reduced mitochondria-mediated LD consumption; (ii) reduced OXPHOS displayed by infected cells, owing to decreased fatty acid oxidation; and (iii) the low rates of ketogenesis displayed by infected animals.
These studies highlight that mammalian LDs constitute an intracellular first line of defense. LDs actively participate in at least two levels of the innate immune response, accumulating and using antibacterial proteins as well as regulating immune cell metabolism. Because widespread resistance to current antibiotics is common among pathogens, understanding the cellular mechanisms that elicit LD-mediated defense may inform future strategies for the development of anti-infective therapies .
