发布时间:2021年11月01日 09:09:25 来源:振东健康网
资讯作者:Weill Cornell Medical College
编辑翻译:奇奇
本文献于2021年10月27日发表在国际期刊《Scientific Reports》上。俄勒冈州立大学的研究人员们揭示了恶性细胞改变其形状以及入侵不同类型组织的迁移方式,有助于了解和预防癌症转移。
俄勒冈州立大学的一项研究揭示了恶性细胞改变其形状以及入侵不同类型组织的迁移方式。
发表在《科学报告》上的这一发现,是了解和预防癌症转移的关键一步。癌症转移是疾病的内部传播,是导致95%癌症死亡的原因。
领导这项研究的俄亥俄州立大学生物物理学家Bo Sun说:“经过数十亿年的进化,细胞已经学会了许多不同的迁移方式。在正常发育和维持健康的生理过程中(如伤口愈合),需要时细胞就会执行特定的迁移程序。然而,在肿瘤的情况下,癌细胞利用这些迁移程序来维持它们对组织的入侵。”
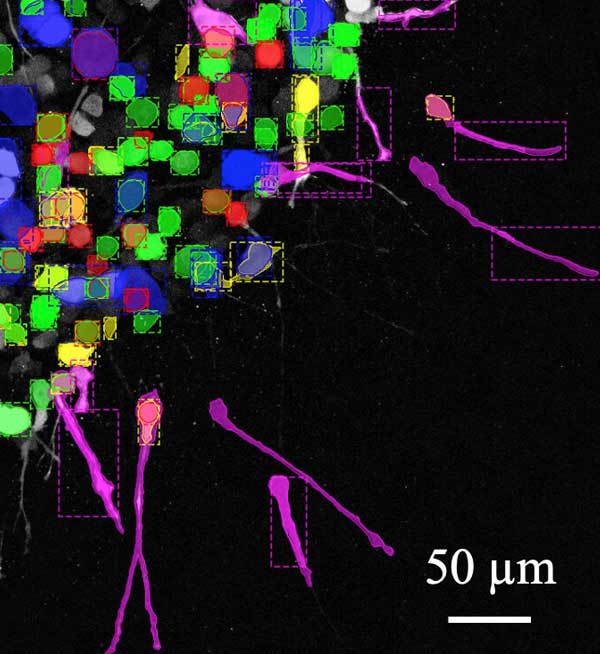
图注:黄色:肌动蛋白。粉色:丝状伪足。绿色:半球气泡。蓝色:板状伪足。红色:小气泡。
孙博士解释说,癌细胞改变形状和移动模式的能力对癌症患者的预后起着巨大的作用。
他说:“许多针对细胞特定移动方式的癌症疗法无法阻止肿瘤转移,很大程度上是因为细胞转向了另一个可用的迁移程序。”
孙博士和他在俄勒冈州立大学科学与工程学院的合作者使用了一种被称为计算机视觉的人工智能,这种技术能根据细胞的形状跟踪细胞的迁移程序,能让计算机视觉从数字照片、视频和其他视觉输入中来获取信息。
在这项研究中,科学家们观察了MDA-MB-23细胞,这是一种常用于医学研究的高侵袭性乳腺癌细胞。孙博士说细胞形状分析就好像根据游泳者的身体姿势和动作来判断游泳者的游泳方式一样。
孙博士说:“细胞形状是由细胞功能决定的,特征形状的丧失与功能异常有关。这就是为什么形状特征一直是诊断癌症以及其他疾病(如红细胞疾病或神经系统疾病)的重要工具。” 他说,研究结果表明,癌细胞改变其迁移模式的频率远比之前认为的要高。
“虽然我们在研究的乳腺癌细胞中看到的不断转换并不一定会使它们在特定类型组织中的速度最大化,但它允许细胞入侵异质组织环境。”孙博士说到。
研究人员指出,在转移过程中,迁移的癌细胞必须通过具有不同力学特性的细胞外基质。细胞外基质(ECM)是组织和器官的非细胞部分。由于其生物活性分子的多样性,它作为一个支架,执行一系列重要的生化和生物力学工作。
科学家们通过机器学习和可视化技术表明,细胞的形状变化是由分子控制中心Rho/rock信号调节的,细胞利用Rho/rock信号感知其物理环境并产生运动所需的力。
通过研究代表两个机械上不同的细胞外基质层的模型,科学家们发现当细胞接近和穿过这两层基质的界面时,它们的形状和运动程序逐渐发生改变。这表明,这些转变对癌细胞转移是必不可少的,这需要非均匀ECM的导航。
孙博士说:“细胞形态变化的方式(形态动力学)是决定其入侵潜力的关键因素,据我们所知,此领域还尚未得到研究。癌细胞迁移的形态动力学正在成为检查细胞内部状态和微环境的强大工具。未来的研究需要将形态动力学解码为丰富和易理解的细胞身体语言,并将影响形态动力学作为一种控制细胞行为的手段。”
英文原文
Research Shows Cancer Cells Change Shape and How They Move to Invade Different Types of Tissue
Oregon State University research has shed new light on the way malignant cells change their shape and migration techniques to invade different types of tissue.
The findings, published in Scientific Reports, are a key step toward understanding and preventing cancer metastasis, the internal spreading of the disease that's responsible for 95% of all cancer deaths.
"Through billions of years of evolution, cells have learned a number of distinct ways to migrate," said OSU biophysicist Bo Sun, who led the study. "In normal development and health-maintaining physiological processes such as wound healing, specific migration programs are executed when required. In the case of a tumor, however, those migration programs are leveraged by cancer cells to sustain their invasion into tissue."
How well a cancer cell can change shape and shift travel modes plays a huge role in a cancer patient's prognosis, Sun explains.
"Many cancer therapies that target a particular way a cell moves can fail to stop tumor metastasis in large part because cells switch to another available migration program," he said.
Sun and collaborators in the OSU colleges of Science and Engineering used a type of artificial intelligence known as computer vision to track a cell's migration program based on its shape; computer vision derives information from digital photos, video and other visual inputs.
For this study, the scientists looked at cells from MDA-MB-23, a line of highly invasive breast cancer cells that's commonly used in medical research. Sun likens the cell shape analysis to determining whether a swimmer is doing the backstroke, breaststroke or butterfly based on the position swimmers put their body in and the movements they execute.
"Cell shape is determined by cell function, and loss of characteristic shape is associated with functional abnormality," Sun said. "That's why shape characterization has been an important tool for diagnosis in cancer as well as in other conditions such as red blood cell disease or neurological disorders." The findings show that cancer cells change their migration modes far more often than had been previously thought, he says.
"While the constant switching we saw in the breast cancer cells we studied doesn't necessarily maximize their speed in a particular type of tissue, it allows for the cells to invade heterogeneous tissue environments," Sun said.
During metastasis, a migrating cancer cell has to make its way through extracellular matrix that has distinct and varying mechanical properties, the researchers note. The extracellular matrix, or ECM, is the non-cellular part of tissues and organs. It acts as a scaffold and, thanks to its variety of biologically active molecules, performs a range of important biochemical and biomechanical jobs.
The machine learning and visualization techniques the scientists employed showed that a cell's shape changes are regulated by the molecular control hub, Rho/ROCK-signaling, that a cell uses to sense its physical environment and generate the force required for motion.
Using a model representing two mechanically distinct layers of extracellular matrix, the scientists showed the cells gradually changed their shape and movement program as they approached and crossed the interface of the layers. That suggests these transitions are essential for cancer cell metastasis, which requires the navigation of non-uniform ECM.
"The way a cell's form changes—its morphodynamics—is a crucial factor in determining its invasive potential and to our knowledge this has largely gone unstudied," Sun said. "The morphodynamics of migrating cancer cells are shaping up to be a powerful tool for inspecting the internal state and microenvironment of the cells. Future research is needed to decode the morphodynamics into a rich and understandable body language of cells, and to affect morphodynamics as a means of controlling what the cells are doing."
参考文献
Christopher Z. Eddy et al, Morphodynamicsfacilitate cancer cells to navigate 3D extracellular matrix, Scientific Reports(2021).
